Helping your clinical lab comply with new FDA regulations
The FDA’s new regulation requires Laboratory Developed Tests (LDTs) to be treated as In Vitro Diagnostics (IVDs). Clinical labs must comply with more stringent FDA guidelines, including premarket approval, quality system regulations, and post-market surveillance.
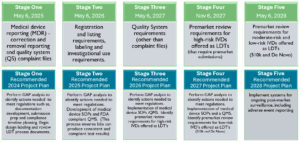
View the FDA’s phaseout policy and our recommended compliance plan.
Our value to your clinical lab:
-
Build a Regulatory Compliance Strategy
Develop tailored strategies to ensure labs meet all new FDA regulations efficiently, including documentation, submission preparation, and compliance timelines.
-
Training and Education
Provide comprehensive training programs for lab personnel on the new regulations, QMS implementation, and best practices for maintaining compliance.
-
Gap Analysis and Audits
Conduct thorough gap analyses and compliance audits to identify areas needing improvement and provide actionable recommendations to address deficiencies.
-
Design labeling and review LDT process documents to meet new FDA labeling compliance.
We’ll design everything and review the process docs to ensure compliance with FDA labeling.
-
Quality Management Systems (QMS) Development
Assist in the development and implementation of medical device SOPs and a QMS to meet FDA’s Quality System Regulation (QSR) requirements. This process ensures labs can produce consistent and compliant test results.
-
Premarket Submission Support
We guide labs through the premarket submission process, including preparation of 510(k), PMA, or De Novo submissions, ensuring all necessary data and documentation are accurately compiled and submitted.
-
Post-Market Surveillance
Set up systems for ongoing post-market surveillance, including adverse event reporting, to ensure continuous compliance and quality improvement.